which type of bond occurs between water molecules
Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other slightly negative ions. All of the electron pairsshared and unsharedrepel each other.
 |
| Chemical Bonds Bio103 Human Biology |
Diamond is a carbon allotrope.
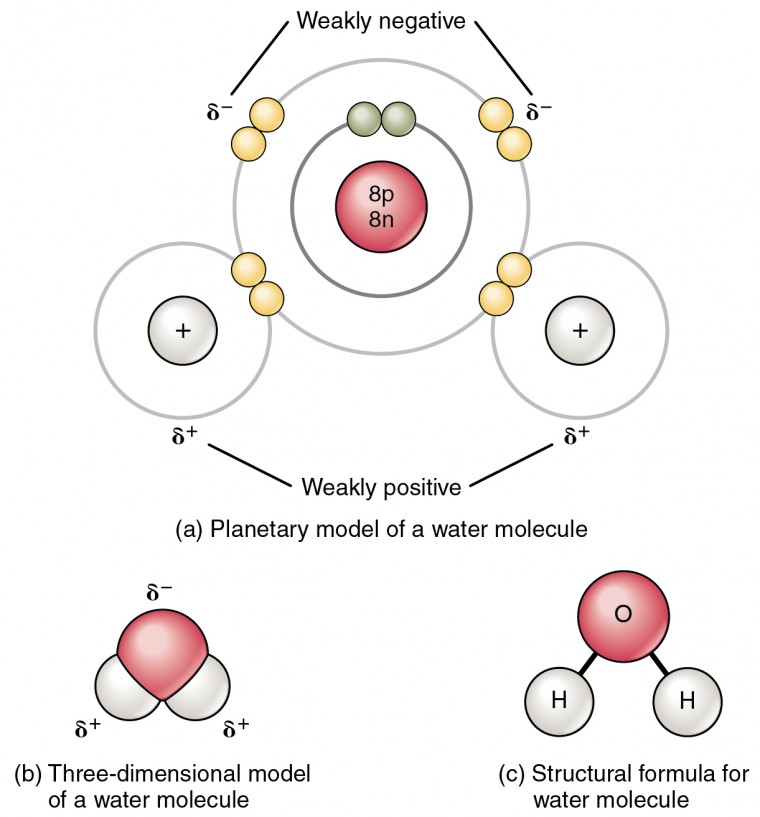
. Hydrogen bonds can form between different molecules and they do not always have to include a water molecule. There are two types of hydrogen bonds found. Which type of bond occurs between water molecules nonpolar covalent metallic hydrogen ionic. Due to the mutual sharing of electrons they developed between two atoms.
The property of cohesion describes the ability of water molecules to be attracted to other water molecules which allows water to be a sticky liquid. Hydrogen bonds are also. In the case of water hydrogen bonds form between neighboring. This shows that two hydrogen atoms and one oxygen atom share electrons with each other to become stable.
Between water molecules hydrogen bonds are found which are called as intermolecular hydrogen bonding The water molecule has H-O-H 2 hydrogen and one oxygen present. Hydrogen bonding between two water H2O molecules. This is because the oxygen atom in addition to forming bonds with the hydrogen atoms also carries two pairs of unshared electrons. Attraction between a positive charge and a negative one between a.
Since both hydrogen and oxygen are non-metals a covalent bond is formed between them. The covalent bond is formed due to the sharing of an electron that occurs between hydrogen H and oxygen O atoms in order to complete their octet shell and hence attains stability. HF water H 2 O molecule. H δ O δ H δ O δ H 2δ O δ H 2.
Which type of bond occurs between water molecules nonpolar covalent metallic hydrogen ionic. Molecules form by two main types of bonds. The covalent link is the most powerful chemical bond. And hydrogen bonding occurs when hydrogen is bound to a strongly electronegative element such as oxygen or nitrogen or fluorine.
What causes a diamonds melting point to be so high. The weak attractive forces that hold water molecules together are called. A hydrogen bond in water occurs between the hydrogen atom of one water molecule and the lone pair of electrons on an oxygen atom of a neighboring water molecule. Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water molecules.
Water is a classic example of a covalent bond because both hydrogen and oxygen atoms exchange electrons. These four hydrogen bonds optimally arrange themselves tetrahedrally around each water molecule as found in ordinary ice see right. Which molecules can form hydrogen bonds with water. Hydrogen Bonds Make Water Sticky In the case of water hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
Hydrogen bonds are the electrostatic attraction ie. The ionic bond and the covalent bond. But the intermolecular bonds the bonds BETWEEN water molecules are the result of hydrogen bonding. The hydrogen bond in water is a dynamic attraction between neighboring water molecules involving one hydrogen atom located between the two oxygen atoms.
The most stable arrangement is the. Water H2O is a covalent compound. A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring. In water a molecule can form up to four hydrogen bonds with one molecule for each hydrogen atom and with two hydrogen atoms on the negative oxygen side.
An ionic bond transfers an electron from one atom to another and a covalent bond shares the electrons. The energy change associated with ionic bonding depends on three main processes. In the water molecule above both O-H covalent bonds are of the normal or conventional type because each atom contributes 1 electron to be shared between the two atoms. Compared to the polar covalent bonds that hold the oxygen and hydrogen atoms together within a molecule of water the hydrogen bonds that hold multiple water molecules together are.
A water molecule consists of two hydrogen atoms bonded to an oxygen atom and its overall structure is bent. Hydrogen bonds are found between water molecules. It occurs between different molecules of a compound and results in increasing solubility in water and a high boiling point. It occurs within different parts of the same molecule and results in decreasing.
Note that the oxygen atom has provided both electrons to be shared with the hydrogen ion so this bond is a coordinate covalent bond. The ionization of an electron from one atom the acceptance of the electron by the second atom and the. Hydrogen atoms in polar bonds within any molecule can form bonds with other adjacent molecules. This is suggested by the formula of water H2O.
For example hydrogen bonds hold together two long strands of DNA to give the DNA molecule its characteristic double-stranded structure. Covalent bonding occurs between non-metals. Because the bond forms between two hydrogens and one oxygen are covalent in nature. In water these bonds are strong but are constantly shifting breaking and re-forming to give water its special properties.
Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule. The O atom is highly electronegative in comparison to the H atom hence it attracts the electron pair towards itself making O atom itself partial negatively charged and H atom partial. The attraction between individual water molecules creates a bond known as a hydrogen bond.
 |
| Hydrogen Bonds In Water Article Khan Academy |
 |
| Chemical Bonds Anatomy And Physiology I |
 |
| The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey |
 |
| Hydrogen Bonding Chemistry For Non Majors |
 |
| Hydrogen Bonding Bioninja |
Posting Komentar untuk "which type of bond occurs between water molecules"